Surfactants in cosmetics - Why better sulfate-free?
What are surfactants actually?
The word "surfactant" comes from the Latin "tensus" and means tension. Because, in general, they reduce the surface tension or interfacial tension of liquids so much that dispersions become possible or easier.
You can represent one of four types[1]:
1. Nonionic surfactants
2. Anionic surfactants
3. Cationic surfactants
4. Amphoteric surfactants
How do surfactants work and how are they structured?
Surfactants are always the same, regardless of their type. They have a hydrophilic (water loving) head and a lipophilic (fat loving) body. In chemistry, the principle that "like dissolves in like", i. Due to their molecular structure, fats dissolve in fats and fat-like substances, they are also said to be non-polar. While water is polar and dissolves polar substances.[2] Surfactants have both polarities, they are in fat, as soluble in water. By this ability one can e.g. Dissolve sunflower oil in water by adding the right surfactant. However, the two liquids do not "dissolve" into one another, they merely become easier to mix and no longer separate into separate layers.[3] The polarity is due to the difference in electronegativity (how much the atoms want to absorb electrons). If this is not very large (e.g., C-H) then an atom is nonpolar. However, if this difference is quite large (e.g., O-H), then the atom is polarized.[4]
A visualization of the functionality of surfactants:
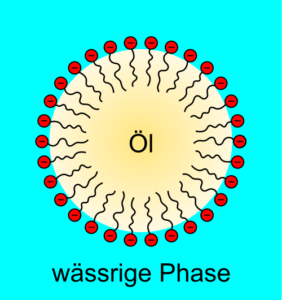
On display are the polar heads (in red with a minus to represent the polarity), with their nonpolar, fat-soluble tails. They surround an area of oil (non-polar) with their tails directed to the oil, and dip their heads into the aqueous phase (polar). Fig. 1
What are the differences between the types of surfactants?
Type 1: nonionic surfactants. These are those surfactants which do not consist of anions and cations, but act on certain functional groups. With them, the electronegativities of the atoms are so great that they are very polar. Such nonionic surfactants usually have several -O- (ether) or -OH (hydroxide) groups. Most surfactants of this type are difficult to degrade or have harmful degradation products. It has been trying for some time to avoid it[5]
Type 2: anionic surfactants. Such surfactants are usually acid-base salts of carboxylic acids and strong lyes or sulfate salts of fatty alcohols. They consist of a charged head and a lipophilic body / tail. Anionic surfactants are often contained in organic, so-called core, and lubricating soaps. These are biodegradable and mainly gentle on the skin. Sulphate Saline surfactants, however, can greatly irritate the skin.[6]
Type 3: cationic surfactants. These are quaternary ammonium salts, which are a bit more complicated. With them, all four valence electrons of nitrogen are bound with one carbon. These conditions give the nitrogen a positive charge which is balanced by a suitable anion (negatively charged particle). These surfactants are biologically well degradable and have as far as possible no negative effects on the skin[7]
Type 4: Amphoteric surfactants. They contain both a positively charged nitrogen cation and a negatively charged carboxylic acid anion or a negatively charged sulfate anion. These are compensated separately by their charge ratio. The ammonium (N++) is balanced by a cation (e.g., Cl–-). And the carboxylic acid or sulfate through a cation (e.g., Na++). These charges contribute to a polarity which acts in both directions, a so-called zwitterion. These surfactants are extremely skin tolerant if they are a -COOH group, less so if they have a sulphate group.[8]
Why are surfactants used in cosmetics at all?
In cosmetics, different active substances, carrier substances and bodying agents are often used. These are often not soluble in each other. Creams are designed to distribute active ingredients on the skin, and require a certain consistency, which can not be created without surfactants. Fluids and serums are designed to concentrate highly concentrated active ingredients, and are best absorbed quickly, in contrast to creams, which are mostly intended to protect and stay on the skin for a longer period of time. Shampoos, toothpaste and soaps are said to purify, condition and refresh, surfactants combine their basic active ingredients, but in addition they serve to remove fat and sebum from the skin and hair, in shampoos and soaps, as well as grind and deposits on the teeth, in Toothpastes, eliminate. Here they bind the, normaly, non-water-soluble particles with water, making it easier to remove them. Without surfactants, cosmetics like we know them would not exist with all their benefits, remedies and improvements.

An unwanted foam bath, due to the escape of extinguishing foam at an airport Fig. 3
Why is there "good" and "bad" within the surfactants?
To say something is only good or bad is very short, and often wrong. Everything has its good and bad sides, our job is to assess the value and balance of these attributes. Has a lot of good sides, but a bad bad, in which one would have to compromise, so is looking for an alternative. Such alternatives are often found in nature, but not always. The highest principle is health and tolerability, in which no compromises are made. Following these two aspects, compromises are made against diminished performance. Or simply to love the health, we renounced to outrageous benefits.
Where you can say no and why?
Surfactants have some aspects as shown above, which also have some effects and side effects. The type 1 surfactants are often poorly biodegradable and more skin tolerant. With the exception of the polysorbate alkylglucoside, which is derived from sugarcane derivatives. The polyalkylene glycol ether e.g. belongs to one of the more dominant nonionic surfactants, the fatty alcohol ethoxylates. They are made using ethylene oxide, which is a descendant of the petroleum industry. [9] Those of type 2 are moderately to good skin-friendly, and biodegradable, with the exception of sulfates, which are very skin incompatible, and not only damage sensitive skin, but can also cause irritation and allergies in people without a history. The Type 3 are difficult to biodegrade, and moderately tolerated by the skin. And those of type 4 are well tolerated by the skin and moderately biodegradable. [11] What to look out for is that you can not make a precise difference between natural and synthetic. Many of these products are considered natural because they occur as such or their educts are of natural origin. One should watch what is sold as natural, because not everything is natural is also always good. Of course, sulfate salt surfactants are harmful in the long run.
Why are sulfate surfactants used at all?
Sulfate surfactants have excellent properties, sometimes foaming and the generation of stable dispersions. They are relatively cheap and can also be used in so-called "hard water" without losing their efficiency as the e.g. Sodium salt surfactants do. The trace elements in water, magnesium and calcium form insoluble salts that have no surfactant properties and can also deposit as lime.[13]
What is the problem with sulfate surfactants?
Sulfate surfactants are irritating, irritating and they can cause allergies. They not only remove grease and dirt, but also healthy skin, making them more susceptible and permeable. Also, these types of surfactants affect the proteins of the skin by denaturing them, i. The proteins, which are long chains of hydrocarbons, amino acids, carboxylic acids as well as sulfates and sulfides, are composed in very precise forms, which is done by intermolecular forces. These forces can be overcome by environmental influences, and thus the protein can be deformed. [14] The best example is a common fried egg, in which the proteins of the liquid and transparent albumen are deformed by heat, and thus become solid and opaque protein. These processes are often difficult to undo, so it is not possible for a chef to "fry" an egg. Chemically it is possible, and our body does it, but this process takes time. [15] The resulting problem is simply explained. Our body is a finely tuned machine, and largely robust against environmental influences. However, for a normal and controlled process many components must harmonize with each other. A deformed protein is like a broken piece in a puzzle, it can not work properly and this whole process is disturbed, such. Supplying the skin with nutrients, or defense against pathogens. This makes the skin more susceptible to redness and irritation.
Why do we only use sulfate-free surfactants?
Ihre irritierenden Eigenschaften und Langzeit Beeinflussung der Haut finden in qualitativer Kosmetik wenig Begeisterung, zumal es viele Alternativen aus der Natur gibt, welche sehr ähnliche positive Aspekte besitzen, und um einiges Verträglicher für die Haut sind. Ebenfalls werden derartige Tenside nicht so gut von der Umwelt abgebaut wie solche, welche der Natur entspringen.[16] Alkylglucoside aus Zuckerrohr oder natürliche Natriumsalze der Fettsäuren von natürlichen Fetten wie Kokosfett, Kakao- und Sheabutter z.B., um ein paar davon zu nennen.
References to the source:
Information on sulfates in cosmetics [13][15][5][6]
More about sodium lauryl sulfate [10][12]
Denaturation of Proteins Explained [15]
Wikipedia [1][2][3][4][7][8][9][11][14][16]
Quellen: Wikipedia